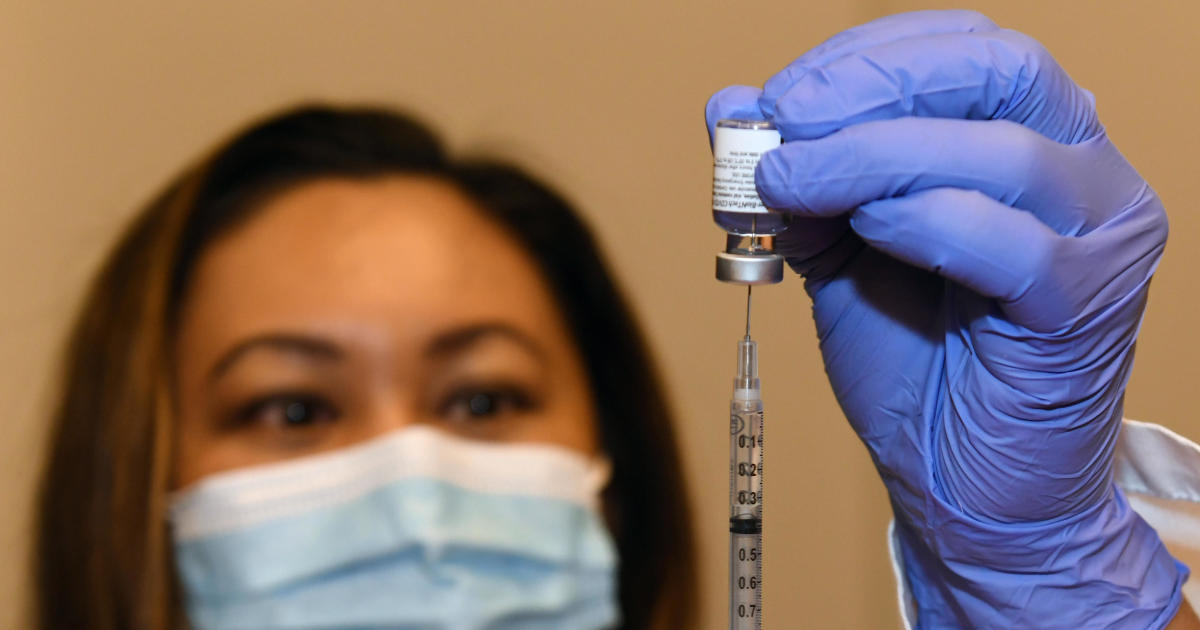
The Food and Drug Administration is expected to authorize Pfizer-BioNTech's COVID-19 vaccine for administration to adolescents by early next week, according to a federal health official with knowledge of the agency's plans.
The move would allow many American middle- and high-school students to be vaccinated against COVID-19 well before the start of the next school year, using a shot Pfizer claims demonstrated "100% efficacy" in children as young as 12 years old with side effects similar to those that have occurred in young adults.
An FDA spokesperson declined to comment on its anticipated approval of an amendment to Pfizer's emergency use authorization, saying only that the regulator was "working to review this request as quickly and transparently as possible."
The New York Times first reported the FDA's plans to expand Pfizer's vaccine authorization to adolescents. Pfizer would be the first COVID vaccine authorized for adolescents in the U.S.
The FDA has previously said it does not plan to convene a meeting of its advisory panel to review the amendment, as it did before first authorizing the vaccines by Pfizer, Moderna, and Johnson & Johnson.
However, the Centers for Disease Control and Prevention will still also need to revise its recommendations for using Pfizer's shot before most providers can begin administering first doses to adolescents. CDC officials have said they plan to quickly convene a meeting of their advisory committee on immunization practices, following the FDA's amendment.
A CDC official told a webinar hosted by the Infectious Diseases Society of America over the weekend that the agency was actively "preparing for" presenting data from Pfizer's trials to the panel of vaccine advisers.
Opening Pfizer's shots to adolescents could provide a surge in demand to a nationwide vaccine campaign that has begun to slow in recent weeks.
More than 21% of doses delivered to jurisdictions have yet to be reported administered, according to CDC figures released Monday, and many states say they are no longer ordering their full allocated supply of doses.
But rolling out the shots over the summer could also pose some challenges to providers.
The CDC currently recommends that the vaccines be administered alone in most cases, more than 14 days before and after any other shots. Federal health officials have repeatedly warned that vaccinations in adolescents had plummeted during the pandemic last year and posed a risk as students anticipate returning to school in the fall.
"We're coming into the summertime when many adolescents come into the office to get their routine vaccines. And so if we missed those then, we may not be able to catch them up in the near future," says Dr. Sean O'Leary, a pediatric infectious diseases specialist at the University of Colorado.
O'Leary acknowledged rolling out the doses to younger recipients could also pose some logistical challenges, though he said that a number of pediatricians have already been successfully giving some doses of COVID-19 vaccine. Pfizer's shot is currently authorized for recipients as young as 16.
"I don't know how many thousands of doses we've given at this point but we, you know, we are ready to go. For the places that have been delivering the 16- and 17-year-olds, I don't think it will be that much of a pivot to start delivering to 12- to 15-year-olds," predicted O'Leary.
Article From & Read More ( Pfizer COVID vaccine for adolescents, boasting "100% efficacy," could be authorized by early next week - CBS News )https://ift.tt/3ue75Qq
Business
Bagikan Berita Ini














0 Response to "Pfizer COVID vaccine for adolescents, boasting "100% efficacy," could be authorized by early next week - CBS News"
Post a Comment